Prioritising the research agenda for retention of participants in clinical trials: PRioRiTy II announces its results
Published: 12 March 2019
By Heidi Gardner
Randomised clinical trials are the gold standard method for gathering evidence about health and care interventions. That said, randomised trials are not perfect, and many of them run into issues through the process of trial design and delivery.
Participants are central to the successful completion of trials; without trial participants researchers would be unable to collect the data needed to answer the question that is central to the trial’s existence. Naturally, that has led to researchers spending significant time and effort on attempting to solve the problem of poor recruitment – the thought process here being that if we can encourage more people to take part in trials, then the likelihood of collecting sufficient data is increased. Unfortunately, once participants are enrolled in a trial, they are not always retained until the trial is complete. Research into reasons behind poor participant retention in trials is limited, and the research community is struggling to get to grips with methods to improve retention as a result.
The UK clinical trials community have previously identified their top research priorities; with methods to boost recruitment identified in first place; and strategies to improve retention and choose appropriate outcomes joint second [1].
The first priority of the UK trials community, ‘research into methods to boost recruitment in trials’, was the subject of the first methodological priority setting partnership (PSP); the Prioritising Recruitment in Randomised Trials study (PRioRiTy), the results of which were published in March 2018 [2]. Following PRioRiTy, a team led by Dr Katie Gillies at the University of Aberdeen’s Health Services Research Unit sought to tackle the problem of research into how to improve retention in trials, and so PRioRiTy II was born. 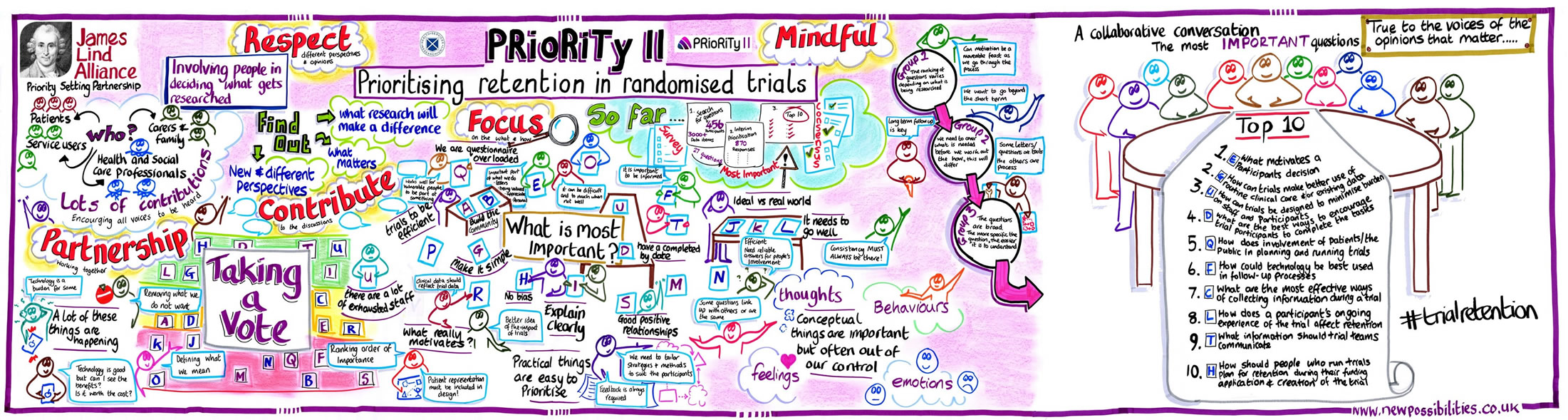
The PRioRiTy II team included many of the team members that had worked on PRioRiTy to share their experiences and to ensure that the methods being used were consistent across both projects. New members were also invite to join the Steering Group to ensure representation across all key stakeholder groups for retention. As previously, JLA representatives also guided the team and ensured due diligence was paid to process.
As with all JLA PSPs, the process started with a broad survey that paved the way for interim prioritisation before a group of relevant stakeholders (in this case that included representation from patients and members of the public, funders, trial methodologists, research nurses, chief investigators, and members of research ethics committees) gathered to produce the final list at a priority setting workshop facilitated by Katherine Cowan, Beccy Maeso and Caroline Whiting from the JLA. The PRioRiTy II workshop was held in Birmingham on Tuesday 23rd October 2018.

Above: The group of stakeholders involved in the PRioRiTy II final priority setting workshop
Dr Gillies (image below) took part in PRioRiTy’s final priority setting workshop to identify priorities for recruitment where she represented the trials methodologist stakeholder group, so leading PRioRiTy II in partnership with JLA was a new experience. After the workshop for PRioRiTy II, Katie said,
“I enjoyed taking part in the PRioRiTy priority setting workshop and was excited to see how the process looked from the other side. I have to say, before working on PRioRiTy II I did not appreciate just how much work Declan and the rest of the PRioRiTy team had invested to get to the final top 10 list! I’m glad that we had their methods and experience to draw on throughout the project, and it’s wonderful to see the top 10 unanswered questions about trial retention be released into the world. Hopefully this will fire up the trial methodology community to conduct research to answer these questions over the coming years.”

Image: Katie Gillies
So, we’ve kept you reading for long enough…
Here are the top 10 unanswered questions about trial retention:
- What motivates a participant’s decision to complete a clinical trial?
- How can trials make better use of routine clinical care and/or existing data collection to improve retention?
- How can trials be designed to minimise burden on staff and participants and how does this affect retention?
- What are the best ways to encourage trial participants to complete the tasks (e.g. attend follow-up visits, complete questionnaires) required by the trial?
- How does involvement of patients/the public in planning and running trials improve retention?
- How could technology be best used in trial follow-up processes?
- What are the most effective ways of collecting information from participants during a trial to improve retention?
- How does a participant’s ongoing experience of the trial affect retention?
- What information should trial teams communicate to potential trial participants to improve trial retention?
- How should people who run trials plan for retention during their funding application and creation of the trial (protocol development)?
What are you waiting for? Let’s get to work answering these questions! We’d love to hear about methodological studies within trials (SWATs) that you are planning to embed into your trials over the coming years.
If you’re stuck for ideas head over to the SWAT repository – a store of SWAT protocols ready to implement into trials. There are currently 5 SWATs registered that focus specifically on retention:
SWAT 48: Effects of a £10 note on trial retention
SWAT 65: Strategies to optimise retention to an online randomised controlled trial for relatives of people with severe mental illness
SWAT 76: Sending pre-notification cards to trial participants before outcome measurement to improve retention
SWAT 79: Effect of birthday cards with or without nudge on retention and data completion rates in trials involving children
SWAT 82: Sending Christmas cards to trial participants to improve retention
You can see a full list of the questions discussed and prioritised at the PRioRiTy II workshop on the JLA website.
An article, What are the most important unanswered research questions in trial retention? A James Lind Alliance Priority Setting Partnership - The PRioRiTy II (Prioritising Retention in Randomised Trials) Study, describes what we did and the results.
References
[1] Tudur Smith, C., Hickey, H., Clarke, M., Blazeby, J., and Williamson, P. The trial methodological research agenda: results from a priority setting exercise. Trials. 2014; 15:32.
[2] Healy, P., Galvin, S., Williamson, P. R., Treweek, S., Whiting, C., Maeso, B., Bray, C., Brocklehurst, P., Clarke Moloney, M., Douiti, A., Gamble, C., Gardner, H. R., Mitchell, D., Stewart, D., Jordan, J., O’Donnell, M., Clarke, M., Pavitt, S. H., Woodford Guegan, E., Blatch-Jones, A., Smith, V., Reay, H., Devane, D. Identifying trial recruitment uncertainties using a James Lind Alliance Priority Setting Partnership – the PRioRiTy (Prioritising Recruitment in Randomised Trials) study. Trials. 2018; 19:147.
